Sublimation Water Cycle Matter And An Experiment
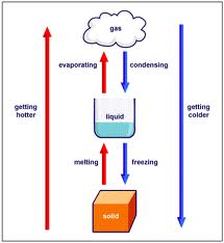
Sublimation The Water Cycle This video teaches about sublimation and the water cycle and sets up an experiment you can easily do at home,. Sublimation is the conversion between the solid and the gaseous phases of matter, with no intermediate liquid stage. for those of us interested in the water cycle, sublimation is most often used to describe the process of snow and ice changing into water vapor (gas) in the air without first melting into water.

Sublimation Water Cycle Matter And An Experiment Youtube 6. rising sea levels. the polar ice caps store the second largest amount of water on earth. (oceans store the most.) the water in the ice caps is in a frozen state and not in motion as part of the water cycle. however, as temperatures increase with global warming, there is melting at the polar ice caps. Watch the process of sublimation the change of state from a solid to a gas using dry ice, hot water, a balloon and lots of suspense. watch the process of sublimation the change of state from. Water ice sublimates under certain conditions: usually at high altitude where the atmospheric pressure is lower and energy from the sun is strong. high winds can cause sublimation. snow on a snowfield sublimates when it is very cold and the sun shines directly on the upper layers of snow. sublimation is part of the water cycle, although it’s. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. an example is the vaporization of frozen carbon dioxide (dry ice) at ordinary atmospheric pressure and temperature. the phenomenon is the result of vapour pressure and temperature.

Comments are closed.