Synthesis Of Aspirin Without Acetic Anhydride
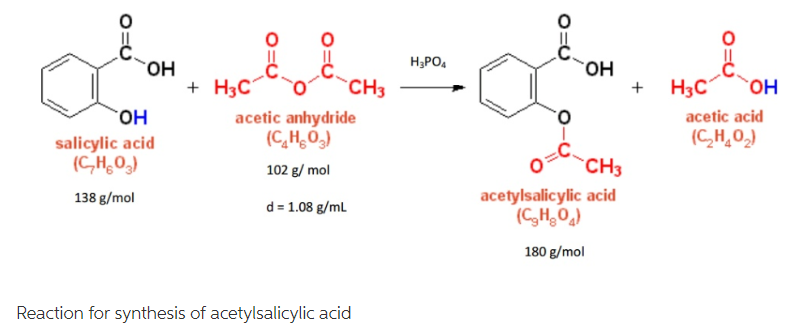
Synthesis Of Aspirin Without Acetic Anhydride Normal method is to react salicylic acid with acetic anhydride, but it is extremely hard to find acetic anhydride. how may i synthesize aspirin with acetic acid and salicylic acid only? i have watched a video where someone makes it with sulphuric acid, salicylic acid and acetic acid. can i use phosphoric acid insted of sulphuric acid as a catalyst?. All answers (6) it is possible to produce aspirin by reacting salicylic acid with acetic acid. this reaction is done in the presence of an acid catalyst and produces an ester. although, the.
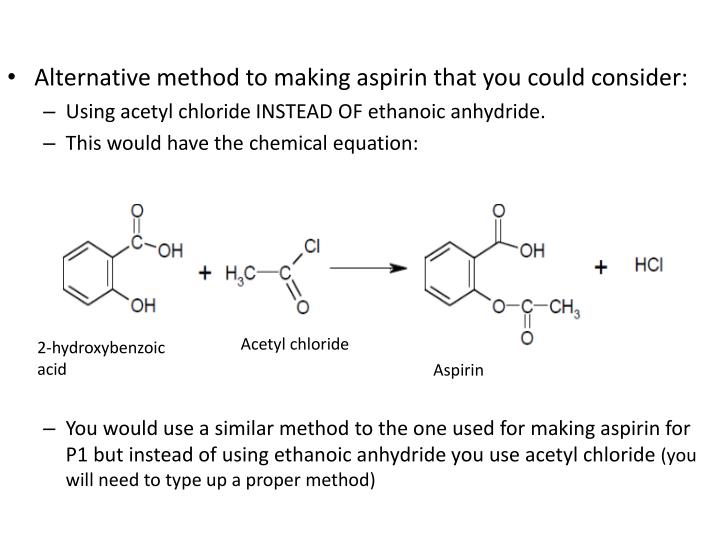
Synthesis Of Aspirin Without Acetic Anhydride Elucidation. for the synthesis of acetylsalicylic acid from salicylic, acid acetic anhydride is usually used as an acetylating agent.the increased reactivity of acetyl chloride is basically not needed here. due to problems in the acquisition of acetic anhydride it is at a minimum of interest to hobby chemists. This reaction replaces the hydroxyl group’s hydrogen atom with an acetyl group, resulting in the formation of acetylsalicylic acid (aspirin) and acetic acid as a byproduct. we use acetic anhydride because it efficiently transfers an acetyl group to salicylic acid’s hydroxyl group. there are a couple of features that make acetic anhydride. With acetic anhydride to form 2 molecules of acetic acid, according to the reaction shown below. when the esterification reaction is complete, water will be added to the mixture. this will cause the precipitation of the acetylsalicylic acid and will react with any remaining acetic anhydride. the solid aspirin will be collected using vacuum. The reaction that is used for the synthesis is shown below. in this reaction, an excess of acetic anhydride (c 4 h 6 o 3) is added to a measured mass of salicylic acid (c 7 h 6 o 3) in the presence of a catalyst, sulfuric acid (h 2 so 4). the mixture is heated to form the acetylsalicylic acid (c 9 h 8 o 4) and acetic acid (c 2 h 4 o 2). after.

Synthesis Of Aspirin Without Acetic Anhydride With acetic anhydride to form 2 molecules of acetic acid, according to the reaction shown below. when the esterification reaction is complete, water will be added to the mixture. this will cause the precipitation of the acetylsalicylic acid and will react with any remaining acetic anhydride. the solid aspirin will be collected using vacuum. The reaction that is used for the synthesis is shown below. in this reaction, an excess of acetic anhydride (c 4 h 6 o 3) is added to a measured mass of salicylic acid (c 7 h 6 o 3) in the presence of a catalyst, sulfuric acid (h 2 so 4). the mixture is heated to form the acetylsalicylic acid (c 9 h 8 o 4) and acetic acid (c 2 h 4 o 2). after. Aspirin (acetylsalicylic acid) belongs to a class of drugs known as nonsteroidal anti inflammatory drugs (nsaids), which work by blocking the synthesis of prostaglandins in the body. before exploring the chemistry of aspirin, one should understand the biological process of inflammation. inflammation in the body occurs in response to the. Synthesis of aspirin (acetylsalicylic acid) place 2.0 g (0.015 mole) of salicylic acid in a 125 ml erlenmeyer flask. add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. h 2 so 4 (use a dropper, h 2 so 4 is highly corrosive) and swirl the flask gently until the salicylic acid dissolves.
Synthesis Of Aspirin Without Acetic Anhydride Aspirin (acetylsalicylic acid) belongs to a class of drugs known as nonsteroidal anti inflammatory drugs (nsaids), which work by blocking the synthesis of prostaglandins in the body. before exploring the chemistry of aspirin, one should understand the biological process of inflammation. inflammation in the body occurs in response to the. Synthesis of aspirin (acetylsalicylic acid) place 2.0 g (0.015 mole) of salicylic acid in a 125 ml erlenmeyer flask. add 5 ml (0.05 mole) of acetic anhydride, followed by 5 drops of conc. h 2 so 4 (use a dropper, h 2 so 4 is highly corrosive) and swirl the flask gently until the salicylic acid dissolves.

Comments are closed.