The Periodic Table Atomic Radius Ionization Energy And Electronegativity

Atomic Radius Periodic Table Neet Lab Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element, including its size and its electronic properties. major periodic trends include: electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. The increasing positive charge casts a tighter grip on the valence electrons, so as you go across the periodic table, the atomic radii decrease. figure \(\pageindex{1}\) shows spheres representing the atoms of the s and p blocks from the periodic table to scale, showing the two trends for the atomic radius.

File Ionization Energy Periodic Table Svg Wikimedia Commons Why is the periodic table arranged the way it is? there are specific reasons, you know. because of the way we organize the elements, there are special patter. Updated on october 06, 2019. use this chart to see at a glance the periodic table trends of electronegativity, ionization energy, atomic radius, metallic character, and electron affinity. elements are grouped according to similar electronic structure, which makes these recurring element properties readily apparent in the periodic table. Periodic table trends. the organization of the periodic table shows the periodic trends of six different physical properties of the elements: atomic radius, electron affinity, electronegativity, ionization energy, and metallic nonmetallic character. atomic radius is half the distance between two identical atoms touching each other. This video explains the major periodic table trends such as: electronegativity, ionization energy, electron affinity, atomic radius, ion size and metallic ch.

Atomic Radius Ionization Energy Periodic Trends Electronegativityођ Periodic table trends. the organization of the periodic table shows the periodic trends of six different physical properties of the elements: atomic radius, electron affinity, electronegativity, ionization energy, and metallic nonmetallic character. atomic radius is half the distance between two identical atoms touching each other. This video explains the major periodic table trends such as: electronegativity, ionization energy, electron affinity, atomic radius, ion size and metallic ch. Interactive periodic table showing names, electrons, and oxidation states. visualize trends, 3d orbitals, isotopes, and mix compounds. fully descriptive writeups. Be the first to ask a question about this topic. 07:53. the periodic table: atomic radius, ionization energy, and electronegativity. professor dave explains. 1. intro to general chemistry 3h 48m. classification of matter. 16m. physical & chemical changes.
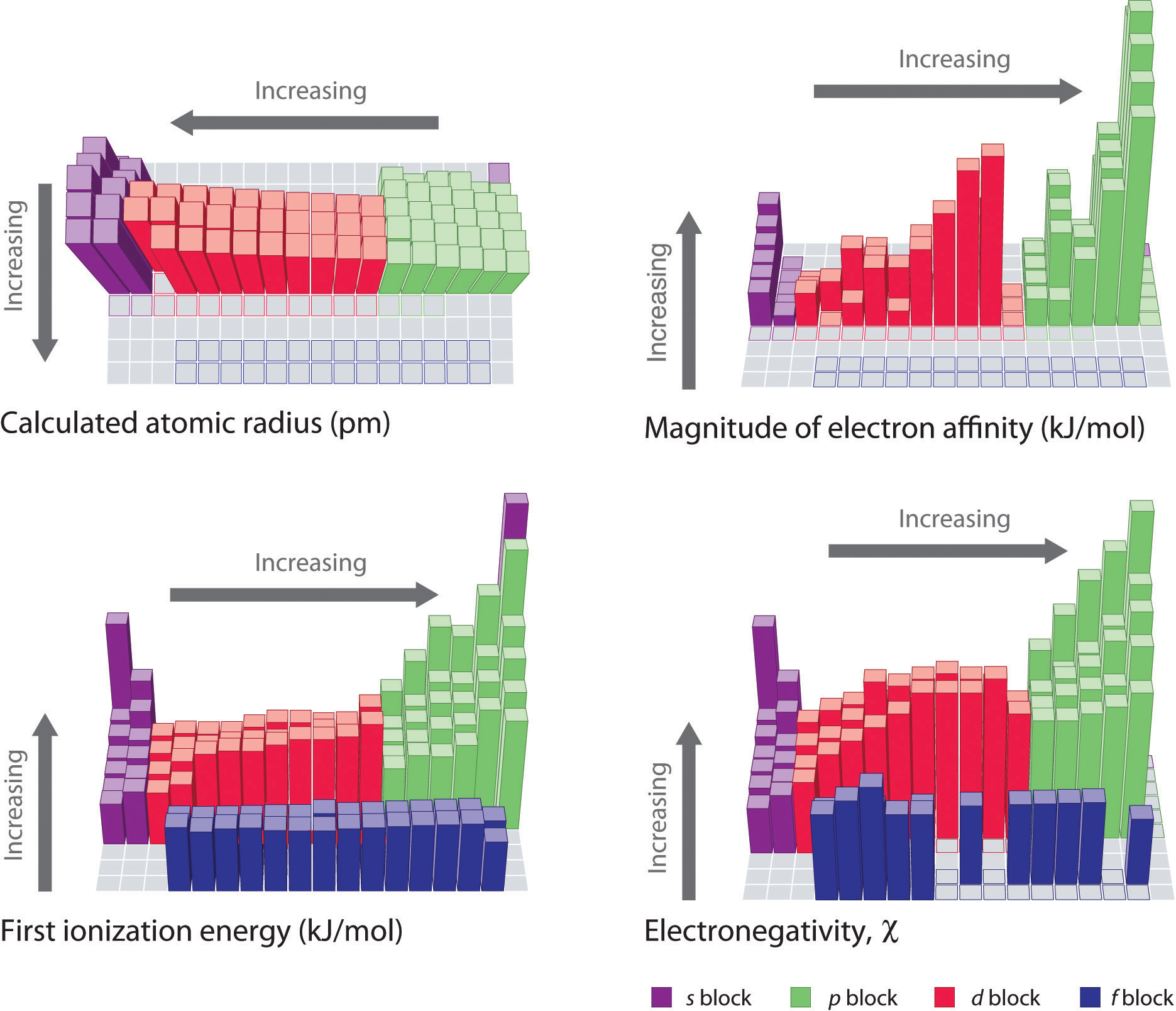
The Periodic Table And Periodic Trends Interactive periodic table showing names, electrons, and oxidation states. visualize trends, 3d orbitals, isotopes, and mix compounds. fully descriptive writeups. Be the first to ask a question about this topic. 07:53. the periodic table: atomic radius, ionization energy, and electronegativity. professor dave explains. 1. intro to general chemistry 3h 48m. classification of matter. 16m. physical & chemical changes.

Comments are closed.