Tiimnottnmnotyyr4s2_1
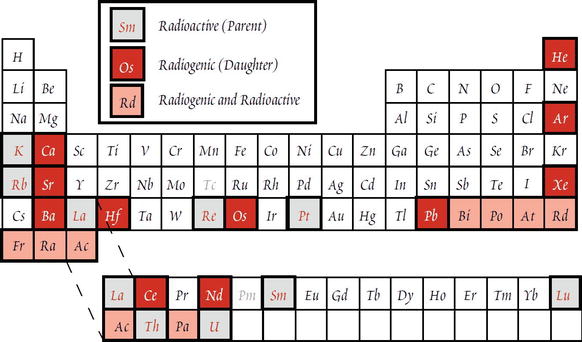
Geochemistry Of Radioactive Isotopes Intechopen Provided to by record uniontiimnottnmnotyyr4s2 1 · sb5t6k66ysb5t6k66y℗ 2020 soundtrack your brandreleased on: 2020 12 14composer, lyricist: linus sjö. A 1 minute timer. use this timer to easily time 1 minutes. fullscreen and free!.
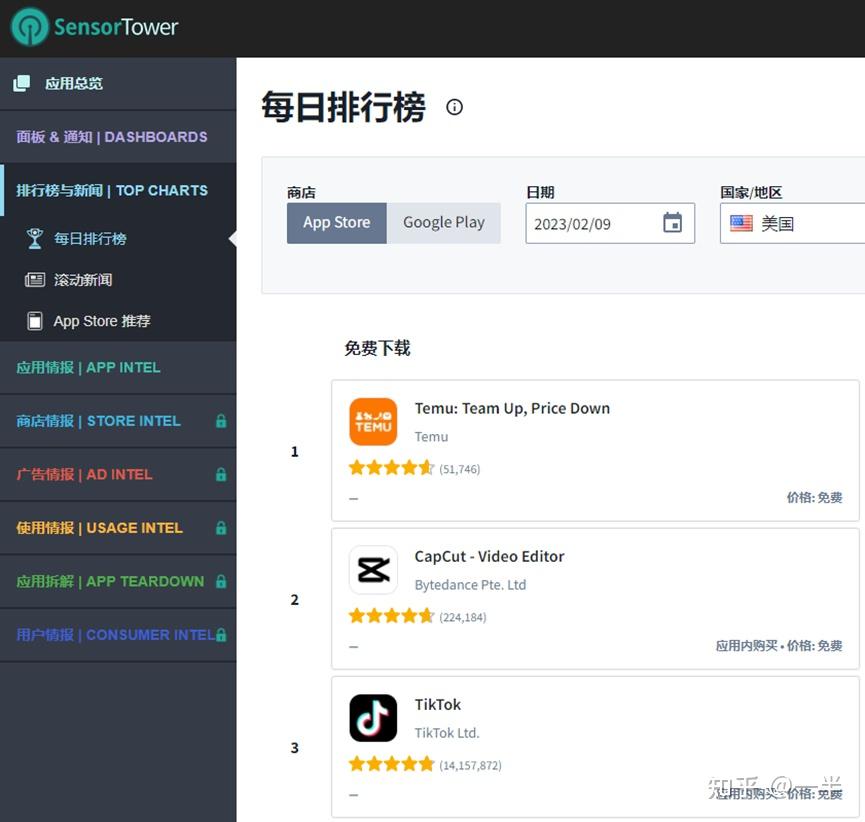
Temu再次登顶北美第一 拼多多这步棋走对了 知乎 Explore math with our beautiful, free online graphing calculator. graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Need a quick and easy way to time your activities? look no further! our 1 minute timer is perfect for timing short tasks or taking quick breaks. [verse 1] i am not okay i'm barely getting by i'm losing track of days and losing sleep at night i am not okay i'm hanging on the rails so if i say i'm fine just know i learned to hide it well. Let us look at the electron configuration for helium, which is 1 s 2 1{\rm s}^2 1 s 2. the first integer, 1 1 1, indicates the primary energy level. in general, its values can be between 1 1 1 to n n n, where n n n is the value of the outermost shell containing an electron. the letter s \rm s s indicates the type of orbital (a.k.a. the subshell).

The Renal Clear Cell Carcinoma Immune Landscape Abstract Europe Pmc [verse 1] i am not okay i'm barely getting by i'm losing track of days and losing sleep at night i am not okay i'm hanging on the rails so if i say i'm fine just know i learned to hide it well. Let us look at the electron configuration for helium, which is 1 s 2 1{\rm s}^2 1 s 2. the first integer, 1 1 1, indicates the primary energy level. in general, its values can be between 1 1 1 to n n n, where n n n is the value of the outermost shell containing an electron. the letter s \rm s s indicates the type of orbital (a.k.a. the subshell). Video 3.1.1 3.1. 1: a trick for writing electron configurations based on the organization of the periodic table. as described earlier, the periodic table arranges atoms based on increasing atomic number so that elements with the same chemical properties recur periodically. The 4s electrons are lost first followed by one of the 3d electrons. this last bit about the formation of the ions is clearly unsatisfactory. we say that the 4s orbitals have a lower energy than the 3d, and so the 4s orbitals are filled first. we know that the 4s electrons are lost first during ionization.

Characterisation Of The Ifds And Dfds Of Nanowires Containing Single Video 3.1.1 3.1. 1: a trick for writing electron configurations based on the organization of the periodic table. as described earlier, the periodic table arranges atoms based on increasing atomic number so that elements with the same chemical properties recur periodically. The 4s electrons are lost first followed by one of the 3d electrons. this last bit about the formation of the ions is clearly unsatisfactory. we say that the 4s orbitals have a lower energy than the 3d, and so the 4s orbitals are filled first. we know that the 4s electrons are lost first during ionization.

Comments are closed.