Trialled And Tested Introducing Clinical Trials

Trialled And Tested Introducing Clinical Trials Youtube What are clinical trials, and why are they so important for answering questions about health?find out more: tv.ndph.ox.ac.uk explore science. Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current.
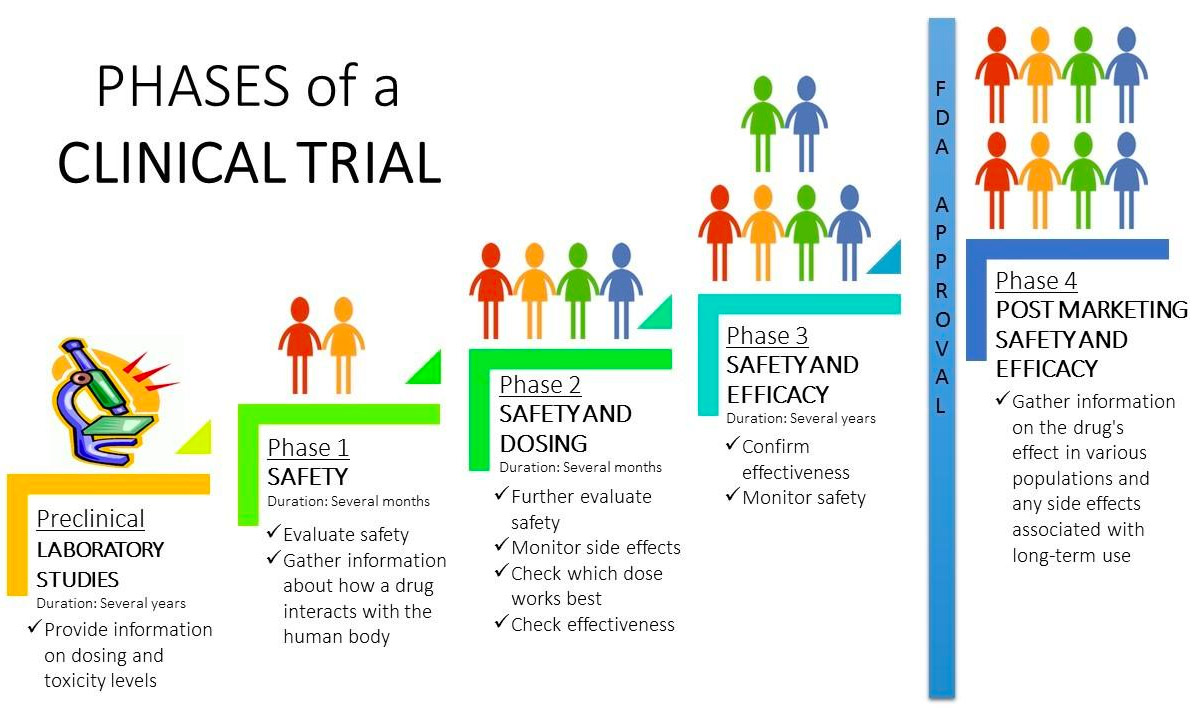
Cancer Clinical Trials Cancer Care St Luke S Cancer Center Clinical research is the study of health and illness in people. there are two main types of clinical research: observational studies and clinical trials. read and share this infographic (pdf, 317k) to learn why researchers do different kinds of clinical studies. observational studies monitor people in normal settings. Clinical trials are organized into a protocol—a detailed plan for how the trial will be conducted. a protocol includes: the length of the trial. information about the treatment being studied. the procedures and tests involved. information about who can participate. rules to be followed. the schedule of the trial’s activities. Clinical trials are designed to answer these questions and improve health and quality of life for patients. until well designed trials have been carried out, we simply do not have enough evidence to know if a treatment is both effective and safe. without trials, there is a risk that people will be given treatments which do not work, and which. Clinical study. a research study involving human volunteers (also called participants) that is intended to add to medical knowledge. there are two types of clinical studies: clinical trial. clinicaltrials.gov identifier (nct number) the unique identification code given to each clinical study upon at clinicaltrials.gov.
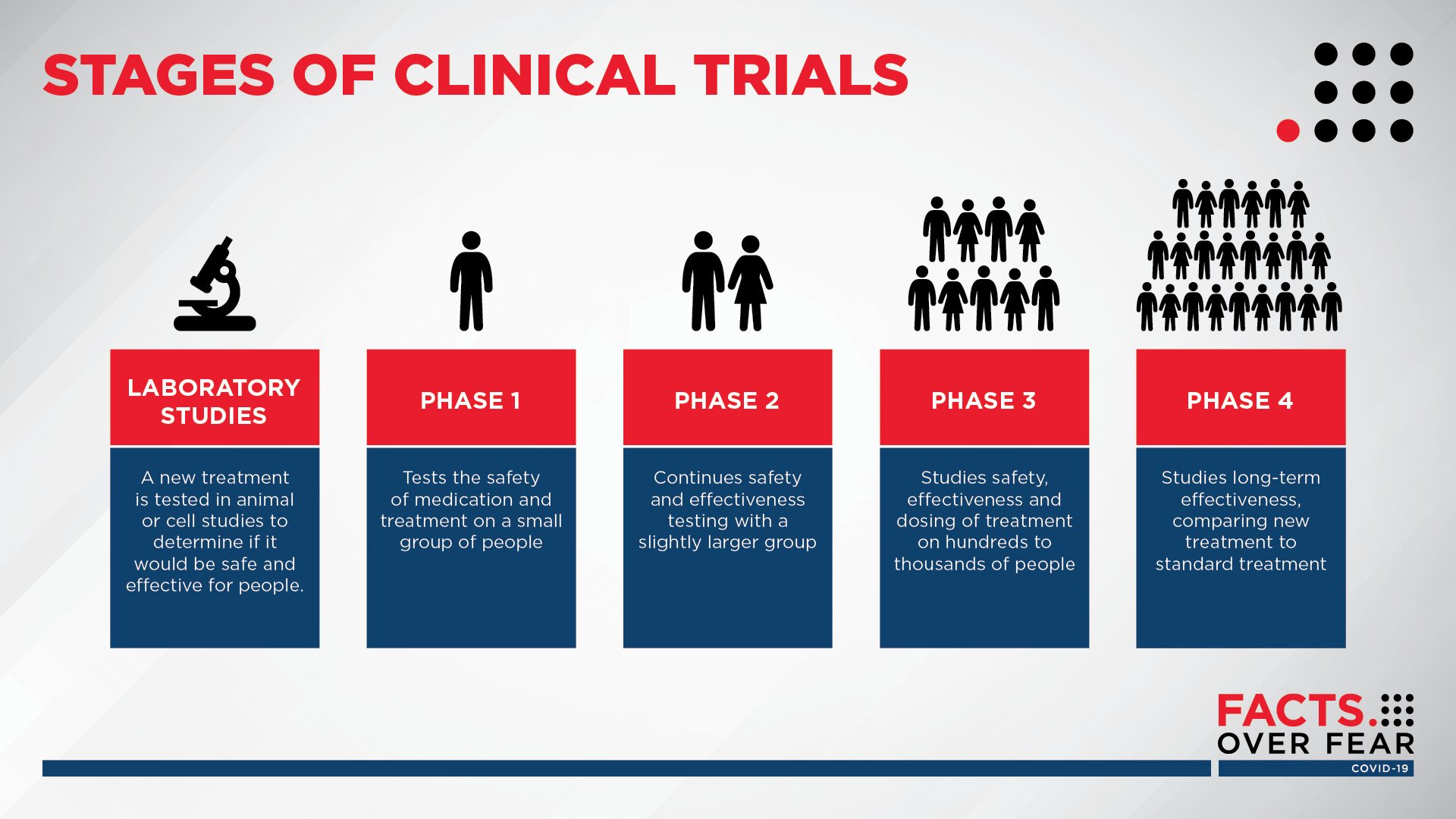
Covid 19 Q A Sanford Research On Clinical Trials Vaccines Sanford Clinical trials are designed to answer these questions and improve health and quality of life for patients. until well designed trials have been carried out, we simply do not have enough evidence to know if a treatment is both effective and safe. without trials, there is a risk that people will be given treatments which do not work, and which. Clinical study. a research study involving human volunteers (also called participants) that is intended to add to medical knowledge. there are two types of clinical studies: clinical trial. clinicaltrials.gov identifier (nct number) the unique identification code given to each clinical study upon at clinicaltrials.gov. While most clinical trials test one alternative to the novel intervention, some expand to three or four and may include a placebo. [citation needed] except for small, single location trials, the design and objectives are specified in a document called a clinical trial protocol. the protocol is the trial's "operating manual" and ensures all. You may hear this process called ‘from bench to bedside’. there is no typical length of time it takes for a drug to be tested and approved. it might take 10 to 15 years or more to complete all 3 phases of clinical trials before the licensing stage. but this time span varies a lot. there are many factors that affect how long it takes for a.

Understanding Clinical Trials Nbdf While most clinical trials test one alternative to the novel intervention, some expand to three or four and may include a placebo. [citation needed] except for small, single location trials, the design and objectives are specified in a document called a clinical trial protocol. the protocol is the trial's "operating manual" and ensures all. You may hear this process called ‘from bench to bedside’. there is no typical length of time it takes for a drug to be tested and approved. it might take 10 to 15 years or more to complete all 3 phases of clinical trials before the licensing stage. but this time span varies a lot. there are many factors that affect how long it takes for a.

Understanding Clinical Trials Nbdf

Comments are closed.