Type Of Reaction For Ch3cooh Caoh2 Cach3coo2 H2o

Type Of Reaction For Ch3cooh Ca Oh 2 Ca Ch3coo 2 H2o Youtube In this video we determine the type of chemical reaction for the equation ch3cooh ca(oh)2 = ca(ch3coo)2 h2o (acetic acid calcium hydroxide).since we ha. Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. ca (oh)2 2 ch3cooh = ca (ch3coo)2 2 h2o. reactants.

Equation For Calcium Hydroxide Dissolving In Water Ca Oh 2 H2o Ca(oh) 2 h 3 po 4; na 2 s 2 o 3 i 2; c 8 h 18 o 2; hydrogen oxygen; propane oxygen; understanding chemical equations. a chemical equation represents a chemical reaction. it shows the reactants (substances that start a reaction) and products (substances formed by the reaction). There are three main steps for writing the net ionic equation for ca(oh)2 ch3cooh = ca(ch3coo)2 h2o (acetic acid calcium hydroxide). first, we balance. Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. 2 ch3cooh ca (oh)2 = ca (ch3coo)2 2 h2o. reactants. In this video we'll balance the equation ca(oh)2 ch3cooh = h2o ca(ch3coo)2 and provide the correct coefficients for each compound.to balance ca(oh)2 ch.

Acid And Base Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. 2 ch3cooh ca (oh)2 = ca (ch3coo)2 2 h2o. reactants. In this video we'll balance the equation ca(oh)2 ch3cooh = h2o ca(ch3coo)2 and provide the correct coefficients for each compound.to balance ca(oh)2 ch. Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. 2 ch3cooh ca (oh)2 = (ch3coo)2ca 2 h2o. reactants. Ca(oh) 2 h 3 po 4; na 2 s 2 o 3 i 2; c 8 h 18 o 2; hydrogen oxygen; propane oxygen; understanding chemical equations. a chemical equation represents a chemical reaction. it shows the reactants (substances that start a reaction) and products (substances formed by the reaction).
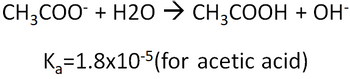
Answered Ch3coo H2o в Ch3cooh Oh вђ Bartleby Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. 2 ch3cooh ca (oh)2 = (ch3coo)2ca 2 h2o. reactants. Ca(oh) 2 h 3 po 4; na 2 s 2 o 3 i 2; c 8 h 18 o 2; hydrogen oxygen; propane oxygen; understanding chemical equations. a chemical equation represents a chemical reaction. it shows the reactants (substances that start a reaction) and products (substances formed by the reaction).

Types Of Chemical Reactions

Comments are closed.