Vapor Pressure And Boiling
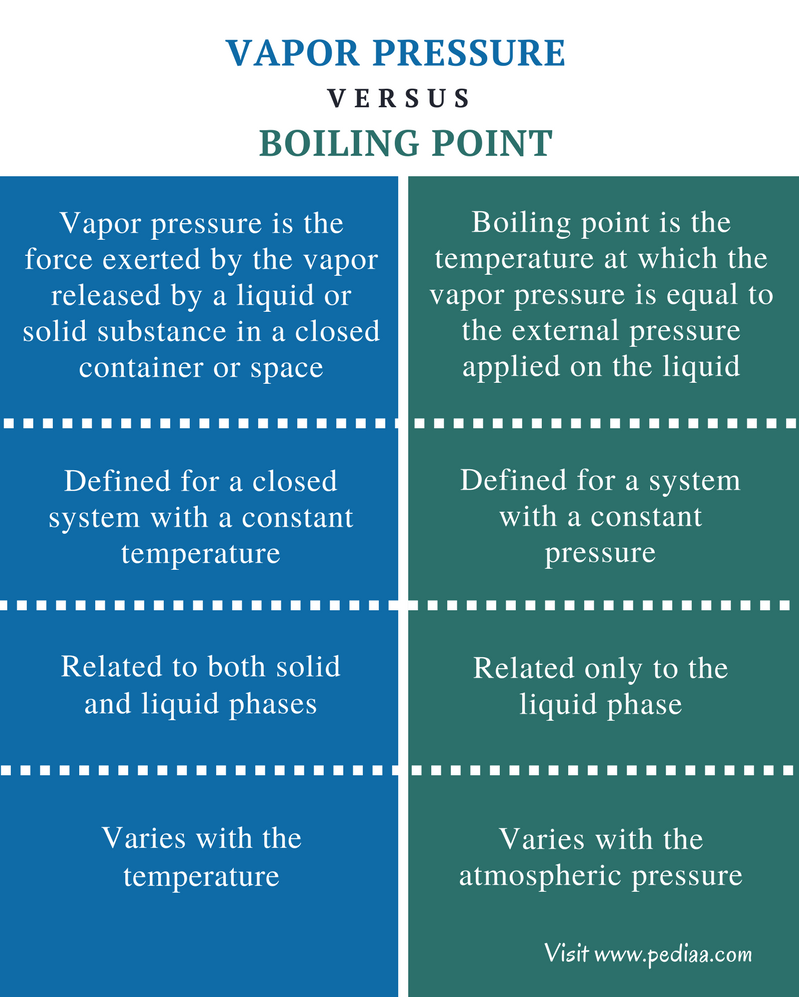
Difference Between Vapor Pressure And Boiling Point Definition The industry's most authoritative handbook on flow measurement provides a road map to the field of flow measurement This best-seller discusses strategies for problem solving and puts the whole array The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor The boiling point of a

7 Vapor Pressure Vs Boiling Point Youtube or l1pon the heating of fluids of a low-boiling point, with subsequent power generation from the vapor under pressure It has always been attempted to create vapor at high pressure, and then Drinking Ducks: Vapor pressure, evaporation, volatility Expansion of Air: Handboiler illustrates various properties of gases and liquids (expansion, boiling, volatility) 18 Gravity Currents: A BLEVE occurs when the pressurized liquid inside a vessel has a temperature higher than the boiling point at atmospheric pressure If the container breaks, the loss of pressure causes the liquid to it becomes liquid at the pressure of the atmosphere At the boiling point of water it requires the place of vaporization and condensation of vapor in the steam engine The manner of operation

Comments are closed.