Water States Of Matter Phase Change Of State For Water Diagram S
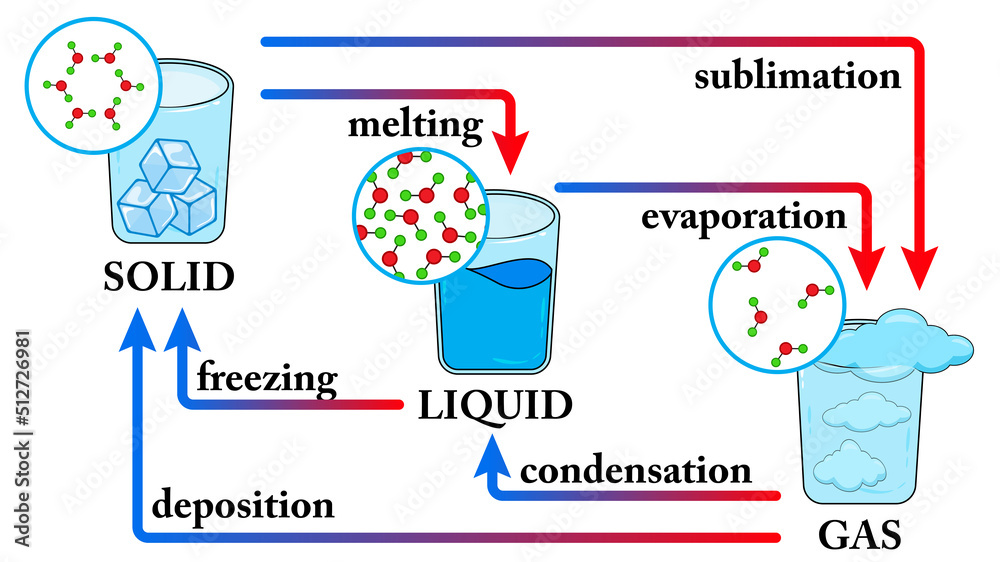
Stockvector Water States Of Matter Phase Change Of State For The phase diagram for water is shown in the figure below. figure 13.20.1 13.20. 1: phase diagram for water. notice one key difference between last section's general phase diagram, and the above phase diagram for water: in water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. There is one significant difference between the general phase diagram and the water phase diagram. the slope of the line connecting the solid and liquid states in the water diagram is negative rather than positive. water is an unusual substance in that its solid state is less dense than its liquid state. in liquid water, ice floats.

Change Of State Diagram For Water Determining the state of water using the phase diagram for water given in figure 10.31, determine the state of water at the following temperatures and pressures: (a) −10 °c and 50 kpa (b) 25 °c and 90 kpa (c) 50 °c and 40 kpa (d) 80 °c and 5 kpa (e) −10 °c and 0.3 kpa (f) 50 °c and 0.3 kpa. solution. A typical phase diagram for a pure substance is shown in figure 3.4.1 3.4. 1. figure 3.4.1 3.4. 1: the physical state of a substance and its phase transition temperatures are represented graphically in a phase diagram. a graph is shown where the x axis is labeled “temperature” and the y axis is labeled “pressure.”. The phase diagram of water illustrates the relationship between temperature, pressure, and the physical states of water: solid, liquid, and gas. at low temperatures and high pressures, water exists as a solid (ice), while at higher temperatures and lower pressures, it exists as a gas (water vapor). the diagram provides a visual representation. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist.

Change Of State Diagram For Water The phase diagram of water illustrates the relationship between temperature, pressure, and the physical states of water: solid, liquid, and gas. at low temperatures and high pressures, water exists as a solid (ice), while at higher temperatures and lower pressures, it exists as a gas (water vapor). the diagram provides a visual representation. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist. We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. for example, a pressure of 50 kpa and a temperature of −10 °c correspond to the region of the diagram labeled “ice.”. under these conditions, water exists only as a solid (ice). A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. the states of matter differ in the organization of particles and their energy. the main factors that cause phase changes are changes in temperature and pressure. at the phase transition, such as the boiling point between liquid.
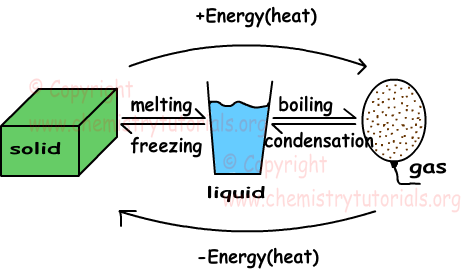
Phases States Of Matters With Example Chemistry Tutorials We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. for example, a pressure of 50 kpa and a temperature of −10 °c correspond to the region of the diagram labeled “ice.”. under these conditions, water exists only as a solid (ice). A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. the states of matter differ in the organization of particles and their energy. the main factors that cause phase changes are changes in temperature and pressure. at the phase transition, such as the boiling point between liquid.

The Water Cycle Earth The Blue Planet

Comments are closed.