What Is The Difference Between Leveling Solvent And Differentiating

Leveling Solvent Vs Differentiating Solvent Tabular Form Functional The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. leveling solvent is the solvent in which total proton transfer happens or the solute is entirely ionized. water (h 2 o) is a popular example of a leveling solvent. a strong acid’s strength is limited (“leveled”) by the. Leveling and differentiating solvents. in a differentiating solvent, various acids dissociate to different degrees and thus have different strengths. in a leveling solvent, several acids are completely dissociated and are thus of the same strength. a weakly basic solvent has less tendency than a strongly basic one to accept a proton. similarly.

What Is The Difference Between Leveling Solvent And Differentiating In a leveling solvent, many acids are completely dissociated and are thus of the same strength. all acids tend to become indistinguishable in strength when dissolved in strongly basic solvents owing to the greater affinity of strong bases for protons. this is called the leveling effect. [citation needed] in a differentiating solvent on the other. Comparative table: leveling solvent vs differentiating solvent. the main difference between leveling solvent and differentiating solvent lies in the way they affect the strength of acids and bases. here is a table comparing the two types of solvents:. The solvent in which the complete proton transfer occurs or the solute is completely ionized is called leveling solvent. one of the most common examples of leveling solvents is water (h 2 o). in such a solvent, several acids are completely dissociated and are thus of the same strength. strong bases are leveling solvents for acids. In conclusion, the difference between leveling and differentiating solvents is that leveling solvents are used to make paints and coatings have a uniform finish, while differentiating solvents are used to create a wide range of colors and effects. leveling solvents are designed to be applied in a thin, even coat, while differentiating solvents.

Differentiate Between Leveling And Differentiating Effect Of Solvent The solvent in which the complete proton transfer occurs or the solute is completely ionized is called leveling solvent. one of the most common examples of leveling solvents is water (h 2 o). in such a solvent, several acids are completely dissociated and are thus of the same strength. strong bases are leveling solvents for acids. In conclusion, the difference between leveling and differentiating solvents is that leveling solvents are used to make paints and coatings have a uniform finish, while differentiating solvents are used to create a wide range of colors and effects. leveling solvents are designed to be applied in a thin, even coat, while differentiating solvents. Difference between leveling solvent and differentiating solvent: leveling solvent differentiating solvent these solvent equals the dissociation of the solute. these solvents dissociate the solute at different degree. for basic solute, acidic solvents act as leveling solvents. for basic solute, weak basic solvents act as differentiating solvents. 2. solvent leveling means that strong acids completely dissociate in that solvent so it is impossible to distinguish between two acids that completely dissociate in it. a solvent levels and acid if the ka k a of that acid is greater than 1. however, that’s not completely dissociated.
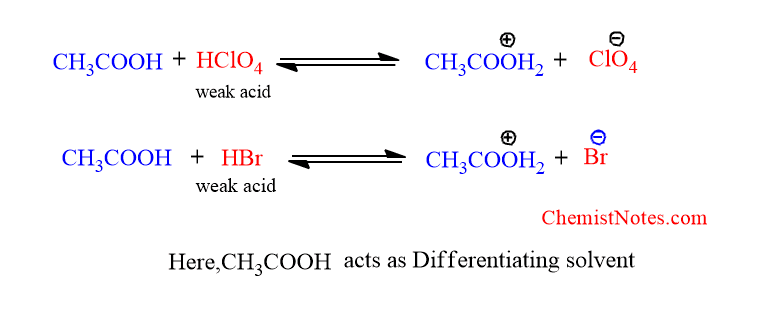
Solvent Leveling Effect Definition Example Chemistry Notes Difference between leveling solvent and differentiating solvent: leveling solvent differentiating solvent these solvent equals the dissociation of the solute. these solvents dissociate the solute at different degree. for basic solute, acidic solvents act as leveling solvents. for basic solute, weak basic solvents act as differentiating solvents. 2. solvent leveling means that strong acids completely dissociate in that solvent so it is impossible to distinguish between two acids that completely dissociate in it. a solvent levels and acid if the ka k a of that acid is greater than 1. however, that’s not completely dissociated.

Levelling And Differentiating Effect Of Solvents Acid Base Chemistry

Comments are closed.