Zolbetuximab Plus Mfolfox6 Successful In Cldn18 2 Positive Subgroup Of
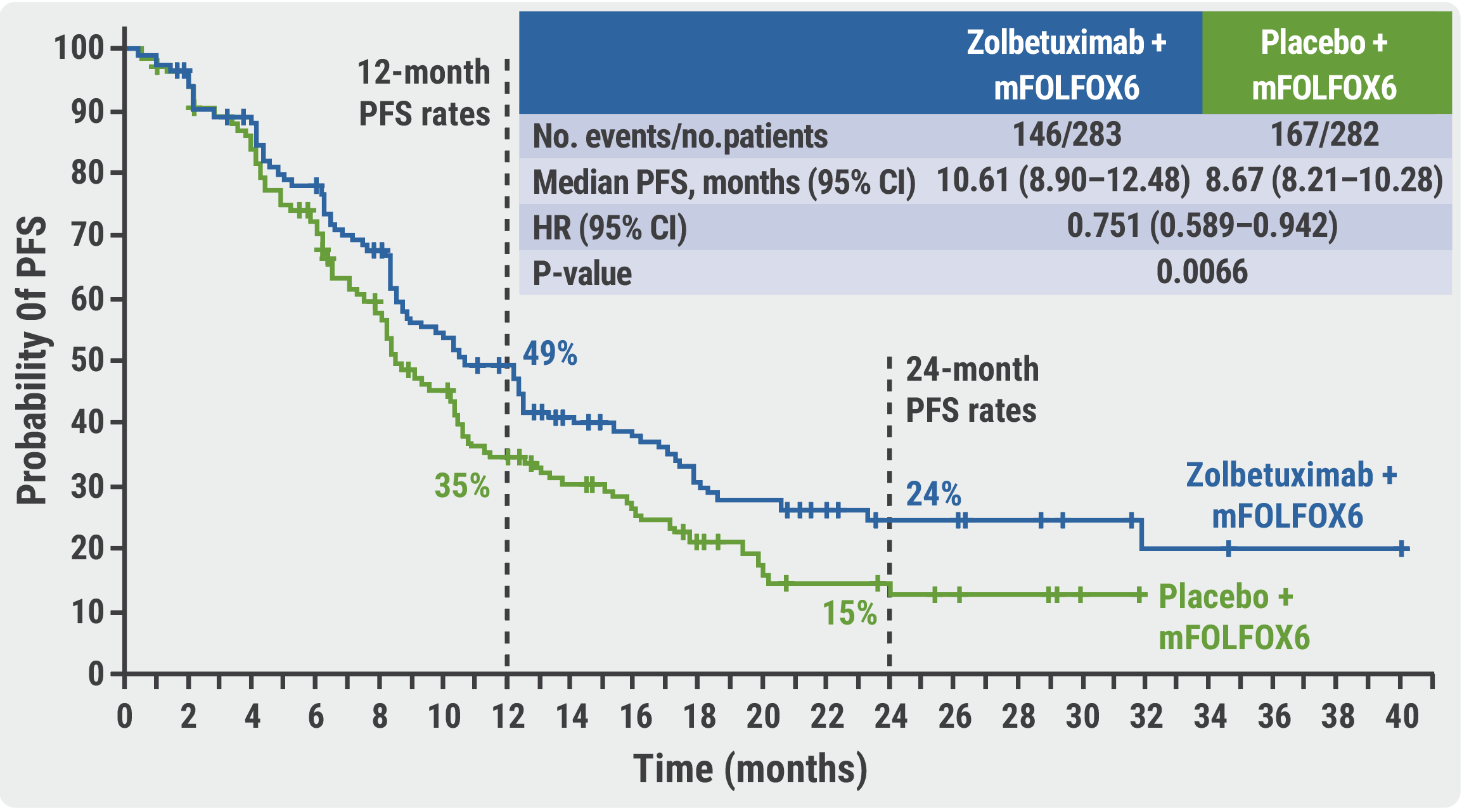
Zolbetuximab Plus Mfolfox6 Successful In Cldn18 2 Positive Subgroup Of We report the results of the spotlight trial, which investigated the efficacy and safety of first line zolbetuximab plus mfolfox6 (modified folinic acid [or levofolinate], fluorouracil, and oxaliplatin regimen) versus placebo plus mfolfox6 in patients with cldn18.2 positive, her2 negative, locally advanced unresectable or metastatic gastric or gastro oesophageal junction adenocarcinoma. Targeting cldn18.2 with zolbetuximab significantly prolonged progression free survival and overall survival when combined with mfolfox6 versus placebo plus mfolfox6 in patients with cldn18.2 positive, her2 negative, locally advanced unresectable or metastatic gastric or gastro oesophageal junction adenocarcinoma. zolbetuximab plus mfolfox6 might represent a new first line treatment in these.

Zolbetuximab Plus Mfolfox6 In Patients With Cldn18 2 Positive He Zolbetuximab is a first in class chimeric immunoglobulin g1 monoclonal antibody that targets and binds to cldn18.2.15, 20 this binding mediates cell death of cldn18.2 positive gastric and gastro oesophageal junction adenocarcinoma cells via antibody dependent cellular cytotoxicity and complement dependent cytotoxicity.20, 22, 23 the phase 2b fast study suggested that zolbetuximab improves. The spotlight trial is a global, randomized, placebo controlled, double blind, phase iii trial designed to evaluate the efficacy and safety of first line zolbetuximab plus mfolfox6 (modified folinic acid or levofolinate, fluorouracil, and oxaliplatin) in patients with cldn18.2 positive, her2 negative, untreated, advanced gastric, or gej adenocarcinoma. 10 cldn18.2 positive was defined as. E16063 background: one accepted treatment for patients (pts) with advanced her2 negative g gej is mfolfox6 (5 fu, folinic acid, oxaliplatin). despite treatment options, 5 year survival is poor, and limited biomarkers exist to inform treatment selection. claudin 18.2 (cldn18.2), a tight junction protein normally confined to gastric mucosa of healthy tissue, is often retained in g gej. The rate of grade 3 or higher adverse events (aes) was 95.2% among patients who received mfolfox6 plus zolbetuximab, 50% in the zolbetuximab monotherapy arm, and 33.3% in the zolbetuximab.

Pdf Cost Effectiveness Analysis Of Zolbetuximab Plus Mfolfox6 As The E16063 background: one accepted treatment for patients (pts) with advanced her2 negative g gej is mfolfox6 (5 fu, folinic acid, oxaliplatin). despite treatment options, 5 year survival is poor, and limited biomarkers exist to inform treatment selection. claudin 18.2 (cldn18.2), a tight junction protein normally confined to gastric mucosa of healthy tissue, is often retained in g gej. The rate of grade 3 or higher adverse events (aes) was 95.2% among patients who received mfolfox6 plus zolbetuximab, 50% in the zolbetuximab monotherapy arm, and 33.3% in the zolbetuximab. Treatment with zolbetuximab and mfolfox6 in the frontline setting resulted in a statistically significantly improved progression free survival and overall survival (os) compared with mfolfox6 in a population with cldn18.2 positive, her2 negative locally advanced unresectable or metastatic gastric gastroesophageal junction (gej) adenocarcinoma, according to a presentation at the 2023. 4036 background: the phase 3 spotlight study showed statistically significant improvements in progression free survival (pfs) and overall survival (os) with 1l zolbetuximab modified folinic acid, 5 fu, and oxaliplatin regimen (mfolfox6) vs placebo (pbo) mfolfox6 in pts with cldn18.2 , her2−, la unresectable or mg gej adenocarcinoma at prespecified interim and later updated analyses. we.

Zolbetuximab Plus Mfolfox6 In Patients With Cldn18 2 Positive He Treatment with zolbetuximab and mfolfox6 in the frontline setting resulted in a statistically significantly improved progression free survival and overall survival (os) compared with mfolfox6 in a population with cldn18.2 positive, her2 negative locally advanced unresectable or metastatic gastric gastroesophageal junction (gej) adenocarcinoma, according to a presentation at the 2023. 4036 background: the phase 3 spotlight study showed statistically significant improvements in progression free survival (pfs) and overall survival (os) with 1l zolbetuximab modified folinic acid, 5 fu, and oxaliplatin regimen (mfolfox6) vs placebo (pbo) mfolfox6 in pts with cldn18.2 , her2−, la unresectable or mg gej adenocarcinoma at prespecified interim and later updated analyses. we.

Comments are closed.